Additional Highlights Include Launch of New $500 Million Hospitality Lending Platform
New York, Jan. 20, 2022 (GLOBE NEWSWIRE) — Madison Realty Capital, a vertically integrated real estate private equity firm focused on debt and equity investment strategies, today announced the completion of one of the most active years in the firm’s 17-year history. 2021’s notable highlights include:
- Closing a record $6.4 billion in total deal volume in 2021 across 72 transactions. The firm executed deals ranging from $10 million to $485 million in all major U.S. metropolitan markets. Throughout 2021, Madison originated and acquired loans across asset classes including multifamily, mixed use, retail, office, industrial, land and hotel and invested in transitional and special situation loans as well as provided financing for ground-up development and construction. In the last two months of 2021 alone, the firm closed 26 new deals representing nearly $2.7 billion.
- Raising $2.08 billion in equity commitments for Madison Realty Capital Debt Fund V LP (“Fund V”), exceeding the fund’s $1.75 billion target. Fund V, the firm’s largest debt fund ever, received significant support from existing investors as approximately 70% of the institutional LPs in Madison’s prior fund re-upped into Fund V. Additionally, 52% of the capital committed for Fund V came from new limited partners, both domestically and abroad.
- Originating over $1 billion in loan-on-loan financing for twelve alternative lenders as part of its lender financing strategy. The firm provided financing solutions to alternative real estate lenders for projects in California, Florida, Nevada, New Jersey, New York, and Oregon through its income-oriented debt investment vehicle, which targets lighter value-add and core-plus real estate transactions with a greater focus on income generation with rates of approximately 4% to 6%.
- Launching an institutional hospitality lending platform, Madison Newbond, with $500 million of initial lending capacity in partnership with Newbond Holdings. Madison Newbond offers unique financing programs to new and existing borrowers across the hospitality spectrum from limited-service hotels and developers to ultra-luxury resorts and targets opportunities including transitional lending and ground up developments, as well as first mortgages, mezzanine loans and preferred equity, across major metropolitan markets.
- Attracting and retaining executive talent. In April 2021, Madison announced seasoned executive Urian Yap joined the firm as Chief Financial Officer from The Blackstone Group, where he led the global loan operations team for Blackstone Real Estate Debt Strategies and the financial reporting team for Blackstone Mortgage Trust Inc. Madison expanded its team with 12 new professionals, further building-out multiple real estate investment disciplines and capabilities. Additionally, Madison, which first opened its Los Angeles offices in 2018, continued to grow its presence on the west coast with the opening of its new Los Angeles office in Century City.
Josh Zegen, Managing Principal and Co-Founder of Madison Realty Capital, said, “Madison Realty Capital further distinguished itself in 2021 by providing single-source, customized financing solutions for borrowers’ unique needs and delivered speed, certainty of execution, and strong underwriting, despite a highly dynamic market environment. I am proud of what we were able to accomplish, which is a testament to our team as well as the culture and expertise we have developed over the past 17 years. We look forward to continuing to execute on behalf of our borrowers, investors, and communities we serve in 2022 and beyond.”
Noteworthy transactions for the firm in 2021 include:
- Breaking ground for a mixed use residential and public school development in Woodside, Queens in a public-private partnership with the NYC School Construction Authority and Department of Education;
- A $34 million loan-on-loan financing for the redevelopment of a multifamily property in Woodland Hills, Los Angeles;
- A $106 million construction loan to Arch Companies and AB Capstone for the ground-up development of Myrtle Point, a mixed-use residence in New York City;
- A $450 million construction loan to The Rabsky Group for a 1,098-unit mixed-use development in Downtown Brooklyn;
- A $278.5 million construction loan to Reger Holdings, LLC for a portfolio of 734 multifamily apartments, 1,264 multifamily units, and 117 luxury condominium residences across three projects in Austin, Texas;
- A $30 million first mortgage loan to Metropica Development for a luxury condominium tower and ten acre development site in Sunrise, Florida;
- A $79 million loan to Vella Group for a portfolio of five industrial and flexible office properties in Los Angeles, California;
- A $395 million loan for a portfolio of 1,161 units across three multifamily projects in Bayonne, Raritan and Linden, New Jersey as well as a land site at the former Bears Stadium with plans for 4,200 residential units;
- A $110 million loan to Harridge Development, Silverpeak Real Estate Partners, and an affiliate of Cerberus Capital Management for single-family homes in a master-planned housing development in Historic San Pedro, Los Angeles.
About Madison Realty Capital
Madison Realty Capital is a vertically integrated real estate private equity firm that, as of December 31, 2021, manages approximately $8 billion in total assets on behalf of a global institutional investor base. Since 2004, Madison Realty Capital has completed approximately $20 billion in transactions providing borrowers with flexible and highly customized financing solutions, strong underwriting capabilities, and certainty of execution. Headquartered in New York City, with an office in Los Angeles, the firm has approximately 70 employees across all real estate investment, development, and property management disciplines. Madison Realty Capital has been frequently named to the Commercial Observer’s prestigious “Power 100” list of New York City real estate players and is consistently cited as a top construction lender, among other industry recognitions. To learn more, follow us on LinkedIn and visit www.madisonrealtycapital.com.
Nathaniel Garnick/Grace Cartwright Gasthalter & Co. +1 (212) 257 4170 madisonrealty@gasthalter.com

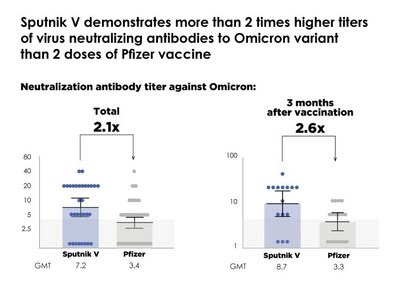
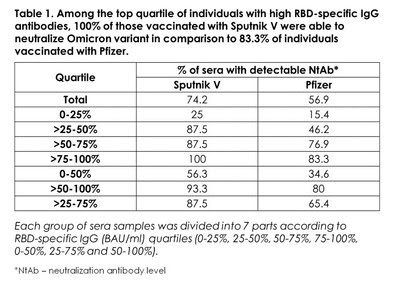
 Partnership among vaccine manufacturers through heterologous (“mix & match”) boosting by other vaccines is needed to address the quickly waning efficacy of mRNA vaccines against COVID-19, which was documented in multiple studies:
Partnership among vaccine manufacturers through heterologous (“mix & match”) boosting by other vaccines is needed to address the quickly waning efficacy of mRNA vaccines against COVID-19, which was documented in multiple studies:
